Why do my results differ between Gen 2 and Legacy columns?
A different resin means a different protocol is required
As Gen 2 columns are built using a new and improved resin that differs to that which was used in Legacy columns, the elution profile will likely differ. We know it differs in our hands (using human plasma) and so we have adjusted the recommended collection protocol to ensure that it is optimised to the new elution profile. Therefore, if you are working with a Gen 2 column but use a collection schedule (buffer volume and Purified Collection Volume (PCV)) optimised for a Legacy column, your Gen 2 column isolation will not be optimal. In fact, it may even look as though it is performing worse than the Legacy columns, purely because the collection protocol is wrong.
We know what it’s like to have a trusty protocol that works, the kind you can almost do in your sleep. Here though, a little adaptation is essential. To take advantage of the increased performance of your Gen 2 column, check the recommended parameters (default buffer volumes, PCV, and guidance on different optimisation scenarios) and elution profiles in your respective qEV Gen 2 User Manual and adjust your elution protocol accordingly.
As shown in Figure 1, the elution profile differs between the Legacy and Gen 2 columns. In this situation, if standard collection parameters for the qEV2 Legacy/ 35 nm (buffer volume 14.1 mL, PCV 8 mL) are used on a qEV2 Gen 2/35 nm column (default buffer volume 17.9 mL, PCV 8 mL), a large portion of EVs will not be collected.
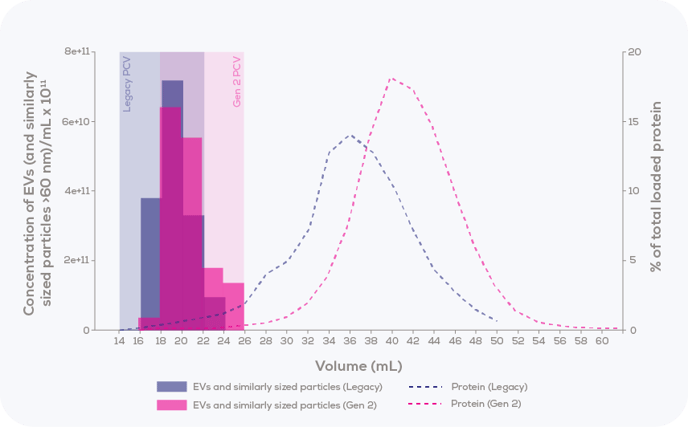
Figure 1: Comparison of elution profiles between the qEV2 35 nm column for Gen 2 vs Legacy, with 2 mL of human plasma loaded. EVs and similarly sized particles >60 nm were measured using the Exoid, and protein was measured via bicinchoninic acid (BCA) assay.
To illustrate the differences that exist between collection parameters for Gen 2 and Legacy columns, values are listed in Table 1.
Table 1: Comparison of Buffer Volumes and Purified Collection Volumes Across Gen 2 and Legacy
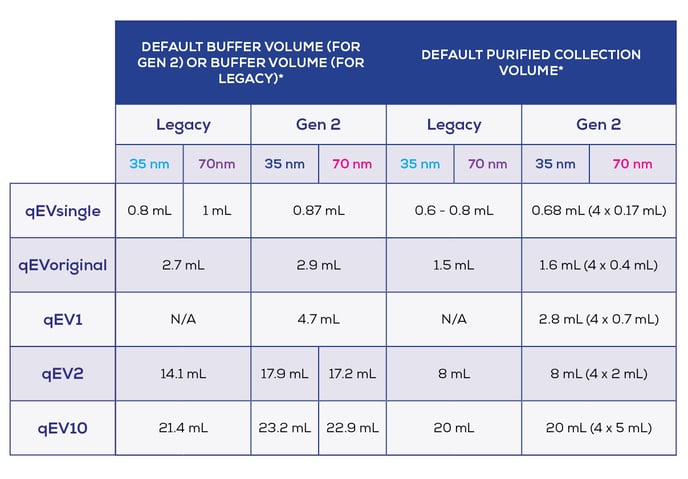
*Note that the values referred to here use a different terminology between Gen and Legacy; elution profiles from Gen 2 columns (human plasma) have been characterised to enable the identification of a default buffer volume, which provides a balance of EV purity and recovery. This default buffer volume differs from the ‘buffer volume’ which is specified separately. Default buffer volumes and default purified collection volumes are programmed onto the Automatic Fraction Collector (AFC). When working with Gen 2 columns, refer to the table in your respective qEV user manual which specifies recommended parameters for different optimisation scenarios.
Read about how EV isolation can be customised using Gen 2 columns
For Legacy qEV columns, the buffer volume and PCV is listed in the respective user manual.
The qEV100 is not compatible with the AFC; refer to your respective qEV100 user manual for guidance on recommended parameters.
The uncomfortable truth
If your differing results were just due to not yet switching to the new protocol, then you can take a sigh of relief. Problem fixed! If, however, you are following the correct Gen 2 protocols and are still getting different results, things might be about to get a bit more complicated.
Gen 2 columns are better at removing contaminant proteins from the EV-rich fractions. Whilst Legacy columns removed ~97% of proteins from the EV-rich fractions, Gen 2 columns remove an impressive ~99%. This difference is small but often crucial. One study has already shown how great qEV Gen 2 columns are at removing contamination (1). Which is great! Unless the proteins, RNAs or even the activity of your EV isolate has changed. In this case, we may have some bad news for you. It could be that some of those proteins, RNAs or bioactivities from your previous EV isolates were due to non-EV contaminants. Even though our Legacy columns were far purer than other EV isolation methodologies, no technique is perfect. Our Gen 2 columns are a little closer to perfect, though. And herein may lie to issue for some customers.
So, how do you know if this is what is happening to you? The simplest way to do this is to follow MISEV2018 guidelines (2) and compare the components and/or bioactivity of the EV-rich fractions with the later protein-rich, EV-depleted fractions. If your expected component or bioactivity is now within the protein fractions, then unfortunately your component/bioactivity was never EV-associated to begin with.
While this might be at first disappointing to discover, there is a silver lining. Just because it isn’t EV-associated doesn’t mean that your discovery is any less important. It’s just a little different.
Still having issues?
In our experience, the two above changes are likely what has caused you to get different results with the Gen 2 columns compared to the Legacy columns. If you are still having issues, please get in touch and our helpful and knowledgeable support team can help you troubleshoot.
References:
- Kronstadt, S. M., Van Heyningen, L. H., Aranda, A. & Jay, S. M. Assessment of anti-inflammatory bioactivity of extracellular vesicles is susceptible to error via media component contamination. Cytotherapy (2023). https://doi.org:10.1016/j.jcyt.2022.12.002
- Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles 8, 1535750 (2019). https://doi.org:10.1080/20013078.2018.1535750